Technology for Modifying the Surface of Clothes and Reducing Washing-related Stress
Fabric Care
The strong mechanical forces exerted on clothes when washing them causes the textiles to snag on each other, causing the clothes to tangle with each other.
If you spin the clothes while entangled instead of separating them, they remain tangled when removed from the washing machine and must be dewrinkled when hung out. This is a washing-related problem that can be quite stressful.
It is known that surfactants have a cleaning function and a surface modification*1 function as well as other different actions depending on how they aggregate in water. When a surfactant is present in the form of spherical aggregates (micelles), it displays cleaning power. When a surfactant forms doubly encapsulated aggregates (vesicles), it attaches more easily to the surface of fabric and can modify its state.
For this reason, conventional surfactants contained in laundry detergents are generally good at cleaning but are not adequately effective in reducing friction or modifying the surface of fabric in other ways. This is because it is difficult to allow a surfactant to form the two different kinds of aggregates—micelles and vesicles—in low concentration ranges like those of laundry detergents during washing.
Kao revealed that an anionic surfactant developed in house (Bio IOS with 18-carbon alkyl chains (C18IOS)) can take advantage of hardness components in water*2 to change the structure of its aggregates in a washing situation.
We further discovered that since this surfactant forms micelles and vesicles in dilute concentrations (in the washing and rinsing processes), it can clean laundry while reducing the friction between the textiles (by modifying the surface).
We will continue to propose new levels of value for laundry detergents by studying the nature of surfactants in our research on interfacial chemistry.
-
* 1 Surface modification refers to changing the nature of the surface of a material and making it lubricative or antistatic
(This function is used in hair conditioners, fabric softeners, and other products). -
* 2 Calcium ions and magnesium ions
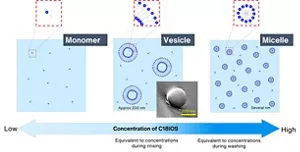
Changes in the way Bio IOS (C18IOS) aggregates in tap water
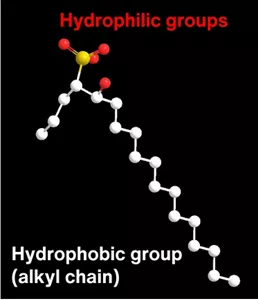
Molecular model of the typical structure of Bio IOS (C18IOS)
- Home
- Innovation
- Research & Development
- Product Development Research
- Fabric and Home Care
- Technology for Modifying the Surface of Clothes and Reducing Washing-related Stress
- Home
- Innovation
- Research & Development
- Product Development Research
- Fabric and Home Care
- Technology for Modifying the Surface of Clothes and Reducing Washing-related Stress
