Successful Direct Observation of Special Bound Water on Cotton Surface Study of Mechanism of Hardening of Wet Cotton Fabric After Natural Drying
This is an English translation of a news release in Japanese on March 18.
A research group formed by the Material Science Research Laboratory of Kao Corporation (President: Michitaka Sawada) and the Institute of Low Temperature Science (Ken-ichiro Murata, Assistant Professor) of Hokkaido University report first direct observations of special water bound to cotton surfaces by use of atomic force microscopy (AFM)*1 and AFM-based infrared spectroscopy (AFM-IR)*2 (Fig. 1). These findings clearly support a model*4 proposed by Kao in 2011, which explains that cross-linking of single fibers*3 mediated by bound water on cotton surfaces causes a cotton hardening phenomenon after natural drying.
This study provides a new perspective for the effects of fabric softener distinct from the established friction reduction theory, and offers insight into better understanding of the structure and function of water on material surfaces, which have recently been topics of much discussion.
The study results have been published in The Journal of Physical Chemistry C.*5
-
* 1 AFM (Atomic Force Microscopy): A probe-type microscope for detecting the force between atoms at the tip of a probe and those of a sample at the atomic level by closely approaching the sample with a pointed probe. This enables observations of a material surface with atomic level accuracy.
-
* 2 AFM-IR (Atomic Force Microscopy-based Infrared Spectroscopy): A highly advanced technique of surface analysis that allows simultaneous direct precise observations of a sample surface and acquisition of its chemical composition/state information. With this technique, a sample surface is irradiated with a tunable IR laser and the resulting photothermal expansion of the surface is measured with the AFM cantilever. IR spectra are obtained by tuning the infrared pulse to absorption wavelengths.
-
* 3 Single fibers: Fine fibers composing a thread.
-
* 4 Refer to the attached English translation of a previously published news release (November 1, 2011: Elucidation of mechanism of the effects of rinse cycle fabric softener)
https://www.kao.com/content/dam/sites/kao/www-kao-com/global/en/news/2011/pdf/20111101-001-01.pdf -
* 5 Direct Observation of Bound Water on Cotton Surfaces by AFM and AFM-IR, The Journal of Physical Chemistry C.
https://pubs.acs.org/doi/10.1021/acs.jpcc.0c00423

Fig. 1. AFM-IR spectra indicating bound water on cotton surface
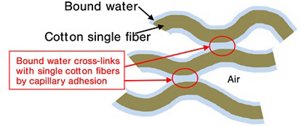
Fig. 2. Schematic illustration of single cotton fibers hardened after wetting and natural drying
Background
In daily life, it is often observed that a cotton towel becomes stiff as if glued when washed without fabric softener and naturally dried. Although common, the mechanism has not been well understood thus far. Kao Corporation has conducted investigations of this hardening mechanism for a long time as part of its research for development of fabric softeners, which led to focused attention given to a special type of water on cotton surfaces, so-called bound water. Bound water is that which binds with a material surface and known to have different molecular mobility from ordinary bulk water, as well as a lower freezing point.
Kao's previous studies and those performed by various measurement techniques*6 have revealed that a certain amount of bound water is adsorbed on the surfaces of naturally-dried cotton,*7 *8 with the bound water showing unique properties different from ordinary water. Based on those findings, Kao has proposed the following model*4 : Cotton exhibits stiffness when naturally dried after wetting as bound water adsorbed on the surface of single cotton fibers causes cross-linking by "capillary adhesion".*9
However, it has not been clarified how bound water is formed on cotton surfaces, why capillary adhesion occurs, how it plays a role in cross-linkage, and whether it exhibits other properties distinct from ordinary water.With the involvement of Dr. Ken-ichiro Murata, Assistant Professor at the Institute of Low Temperature Science, Hokkaido University, who has been conducting cutting-edge studies concerning special-state water on surfaces and at interfaces with materials, a more direct and molecular level technique was used to examine the model.
-
* 6 Dielectric relaxation measurements, calorimetry, neuron scattering measurements, etc.
-
* 7 Approximately 8% of water content exists on a cotton surface (at room temperature of 25˚C and 80% humidity).
-
* 8 Cotton is a highly hydrophilic material possessing a hydroxyl group in the molecular structure, termed cellulose. Residual water after natural drying is considered to be strongly adsorbed into cotton.
-
* 9 A phenomenon that causes adhesion to be mediated by liquid sandwiched between solid surfaces.
Results
In the present study, to obtain direct observations of bound water on cotton surfaces, AFM and AFM-IR, the latter a cutting-edge surface analytical technique that enables high-precision observations of the surface of a sample and acquisition of its chemical composition/state information (infrared absorption spectra), were employed.
1. AFM observations
A large force was observed on the cotton side with detachment when approaching and removing the probe from cotton surfaces. This phenomenon demonstrated the existence of "a viscous substance" on the cotton surface because such adhesion behavior could not be explained only by the presence of cellulose, a major component of cotton. This suggests that such viscous bound water is involved in capillary adhesion of single fibers.
2. AFM-IR observations
AFM-IR spectra of naturally dried cotton surfaces showed two peaks corresponding to the vibration mode of water molecules (Fig. 1). On the other hand, no peaks were observed on the cotton surface after completely removing water. This indicates that these peaks came from bound water existing on the surface. Furthermore, the spectra, showing two clear peaks, significantly differed from those of ordinary water and are thought to reveal bound water in two different states on the surface, possibly corresponding to bound water at the air-water interface and that at the water-cotton interface, respectively.
As shown by results noted in 1 and 2 above, it was experimentally clarified that bound water was evident on cotton surfaces and contributed to dynamic properties such as stiffness mediated by capillary adhesion. In addition, the bound water itself manifested a unique hydrogen bonding state different from that of ordinary water.
Future prospects
In the present study, by focusing on capillary adhesion of bound water, the origin of the phenomenon of cotton hardening when dried naturally after wetting was revealed. These findings clarified that the state of bound water (hydrogen bonding state) is different from that of ordinary water, due to the influence of the cotton surface. It was thus speculated that adhesion between single fibers and the viscosity of bound water are deeply involved not only in the hardening phenomenon, but also in the dynamic property of cotton related to feel against skin.
Furthermore, from the standpoint of application, the results of the present study obtained by elucidation of a part of the mechanism of the cotton hardening phenomenon suggest a new perspective regarding "how to improve the function of fabric softeners".
Recently, along with progress in a variety of surface analytical techniques, the specific structure and function of "water on the surface of a material" have attracted considerable attention. The findings obtained in the present study provide effective clues for better understanding of this phenomenon.
About Kao
Kao creates high-value-added products that enrich the lives of consumers around the world. Through its portfolio of over 20 leading brands such as Attack, Bioré, Goldwell, Jergens, John Frieda, Kanebo, Laurier, Merries and Molton Brown, Kao is part of the everyday lives of people in Asia, Oceania, North America and Europe. Combined with its chemical division, which contributes to a wide range of industries, Kao generates about 1,500 billion yen in annual sales. Kao employs about 33,000 people worldwide and has 130 years of history in innovation. Please visit the Kao Group website for updated information.
Media inquiries should be directed to:
Corporate Communications
Kao Corporation
