Prediction of Complex Developmental Toxicity by Using Non-animal Models
Predictive Toxicology
Ensuring the safety of products requires evaluating their potential effects on both current and future generations.
Kao is actively developing alternative methods to animal testing that can be used for predicting possible impacts on the future generations.
Due to the complexity of embryonic development, accurately predicting developmental toxicity through alternative methods remains a significant challenge.
Kao addresses this by employing an integrated approach to toxicity assessment. This approach combines in silico (computer simulation) techniques for predicting toxicity based on the structure of a substance and existing safety information, with in vitro techniques for precise analysis of substance effects.
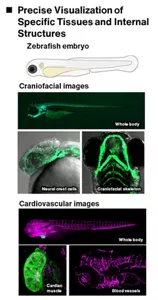
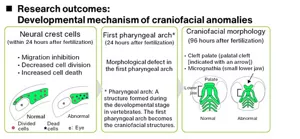
We are attempting to establish a way to quickly and accurately predict effects using zebrafish early embryos. Using confocal microscopy, we can visualize internal structures—such as craniofacial and cardiovascular anomalies—whose subtle morphological changes are difficult to detect in wild-type zebrafish embryos. Kao has developed a system that can not only identify these anomalies but also allows for quantitative and precise evaluation. Moreover, we employ next-generation RNA sequencing to comprehensively analyze alternation in gene expression, providing deeper insights into the molecular mechanisms behind these morphological defects.
This knowledge will contribute to establishing a new for international safety assessment framework through collaboration with f ICCS* and other industry groups and chemical regulators in and outside Japan.
-
* International Collaboration on Cosmetics Safety

- Home
- Innovation
- Research & Development
- Fundamental Research
- Safety Science
- Prediction of Complex Developmental Toxicity by Using Non-animal Models
- Home
- Innovation
- Research & Development
- Fundamental Research
- Safety Science
- Prediction of Complex Developmental Toxicity by Using Non-animal Models
