EpiSensA Alternative Method for Skin Sensitization Testing Developed by Kao Added to the OECD Test Guidelines*1
EpiSensA (Epidermal Sensitization Assay), developed exclusively by Kao Corporation, was adopted in the OECD Test Guidelines, a compendium of globally recognized official test methods, as an alternative method for skin sensitization testing on June 25, 2024*2 . This method makes it possible to evaluate a broad range of chemicals, including lipophilic chemicals which up to now have been difficult to assess using existing alternative methods. EpiSensA is the world’s first alternative method for skin sensitization testing using the artificial skin model*3 to be adopted in the Guidelines. It is the second such testing method to be adopted in the Guidelines, following h-CLAT*4 developed jointly by Kao and Shiseido Company, Limited, which was adopted in the Guidelines in 2016.
To disseminate this technology more widely, Kao waived its exclusive patent*5 to EpiSensA technology in August 2024. This will make possible broader use of the technology worldwide.
-
* 1 Test methods designated by the OECD (Organization for Economic Co-operation and Development) and internationally agreed for use in evaluating the safety of chemicals.
-
* 2 OECD TG 442D https://doi.org/10.1787/9789264229822-en
-
* 3 The LabCyte EPI-MODEL 24 from Japan Tissue Engineering Co., Ltd. (J-TEC) is the only model available for EpiSensA (as of June 2024).
-
* 4 human Cell Line Activation Test
Kao News Release, September 2, 2016: Skin Sensitization Test “human Cell Line Activation Test (h-CLAT)” Adopted as an OECD Test Guideline -
* 5 Japanese Patent No. 6031221 (Patents are only obtained in Japan and not outside Japan)
EpiSensA alternative method for skin sensitization testing
EpiSensA, which focuses on antioxidant and inflammatory responses in the keratinocytes upon exposure to skin sensitizers, is a testing method for evaluating skin sensitization potency based on marker gene expression response to a test chemical applied to the surface of an artificial skin model. Other alternative methods involve dissolving the chemical to be evaluated in a culture medium (aqueous solution), which could not be used for lipophilic chemicals. To overcome this limitation, Kao began working on developing EpiSensA, a next-generation alternative testing method using the artificial skin model, in 2011.
The artificial skin model used in EpiSensA has a structure similar to that of human skin, allowing it to imitate the chemical application as if on human skin (Figure 1). EpiSensA also allows evaluation of lipophilic chemicals, which were difficult to assess using existing alternative testing methods, and is expected to make significant contributions to the development of new chemicals in various industries.
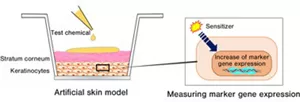
Figure 1. Representation of EpiSensA testing
Ongoing work in animal-free testing and future prospects
Kao aims to develop and disseminate animal-free technologies capable of accurately evaluating skin sensitization. In 2016, the company focused on the in vitro replication of immune cell functions, and together with Shiseido, jointly developed the h-CLAT alternative testing method, which was adopted in the OECD Test Guidelines. This time, EpiSensA, a next-generation alternative method that has overcome various obstacles, underwent validation study*6 by JaCVAM*7 and became the world’s first alternative method for skin sensitization testing using the artificial skin model to be adopted in the Guidelines.
Going forward, Kao will continue to explore other alternative methods for reproducing the sensitization process phenomenon. Additionally, the company will work together with the International Collaboration on Cosmetics Safety, to develop, standardize and disseminate alternative evaluation systems combined with EpiSensA.
-
* 6 Testing by several facilities to confirm the testing method’s utility and reproducibility
-
* 7 Japanese Center for the Validation of Alternative Methods
This is an English translation of reference material released in Japanese on July 31.
For more information:
Media inquiries should be directed to:
Public Relations
Kao Corporation
