Kao's Case Studies on Systemic Toxicity of Chemicals Are Adopted as an International Project Sponsored by the Organization for Economic Co-operation and Development (OECD):
Initiative to Develop Methods That Can Replace Animal Testing
Kao Corporation (President: Michitaka Sawada) has been conducting research on methods for evaluating the safety of chemicals as alternatives to animal testing at its Safety Science Research Laboratories. Recently, two of Kao's case study reports on the evaluation of systemic toxicity*1 of chemicals that the company had conducted without animal testing were approved as one of the OECD's IATA Case Studies Project and were published on the organization's official website on October 1, 2020*2. The purpose of this Project is to accumulate case studies on the evaluation of chemical safety, and to issue guidance on novel evaluation methods. The aforementioned two reports compiled by Kao are expected greatly to help the OECD issue guidance on new alternative methods to animal testing in the Project. This marks the first time that a single private enterprise's case study report was submitted to and adopted by the OECD's Project.
Kao will continue researching alternative methods to animal testing in collaboration with various government agencies and research institutions in Japan and around the world, with the aim of helping consumers lead safe and worry-free lives and also contributing to the advancement of various related industries.
-
* 1 Systemic toxicity means the toxicity of chemicals that affect various organs of the entire human body when exposed to such chemicals.
-
* 2 IATA Case Studies Project, Review year 2019, No. 3-4.
Kao's Research on Alternative Methods to Animal Testing
To ensure the safe use and management of chemicals, it is crucial to evaluate their safety first. In recent years, there has been a growing global need to develop alternative safety evaluation methods that can replace animal testing.
As various chemicals are involved in its consumer goods and industrial chemicals business, Kao started conducting research early on chemical safety evaluation methods that could replace animal testing. As a result, certain skin sensitization and eye irritation evaluation methods, which Kao was involved in developing, as alternatives to animal testing have been incorporated*4-5 into the OECD Guidelines for the Testing of Chemicals*3, which are internationally recognized and accepted testing specifications.
Meanwhile, because it is more difficult to evaluate systemic toxicity compared to local toxicity specific to certain sites such as the skin and eyes largely due to the more complex mechanism through which toxicity is manifested, evaluation of systemic toxicity using a single alternative method to animal testing is considered extremely difficult. Kao started developing systemic toxicity evaluation methods as alternatives to animal testing as early as in 2013.
-
* 3 The OECD Guidelines for the Testing of Chemicals are sets of official testing methods for evaluating chemical safety that are specified by the OECD and agreed to internationally.
-
* 4 Alternative skin sensitization testing method (TG 442E:h-CLAT) adopted by the OECD in 2016.
September 2, 2016 (news release): h-CLAT, an alternative skin sensitization testing method, has been incorporated into the OECD Guidelines for the Testing of Chemicals.
https://www.kao.com/jp/corporate/news/rd/2016/20160902_002/ -
* 5 Alternative eye irritation testing method (TG 491:STE) adopted by the OECD in 2015, revised in 2020.
November 27, 2015: Alternative eye irritation testing method involving STE assay, which has been independently developed by Kao, is approved for inclusion in the OECD Guidelines for the Testing of Chemicals.
https://www.kao.com/jp/corporate/news/rd/2015/20151127-001/
Kao's Systemic Toxicity Case Studies Have Been Adopted in the OECD's International Project
Kao conducted systemic toxicity evaluations of two model chemical groups (chlorobenzenes and alkylphenols) without animal testing, and submitted two case study reports to the OECD IATA Case Studies Project. After the scientific validity of the reports was discussed and verified at the OECD's international conference held on November 19 and 20, 2019, the case studies were approved by the OECD and were published on its official website on October 1, 2020.
In these case studies, Kao evaluated the chemicals using read-across as one of the Integrated Approaches to Testing and Assessment (IATA) based on related toxicity mechanisms. Read-across is a method of inferring the toxicity of a target chemical from the already-available safety data of a data-rich chemical that has a similar enough structure to that of the target chemical. Because it offers the advantage of being able to avoid new animal testing, scientists have worked hard to use read-across in the safety assessment of chemicals instead of animal experiments, but often encountered situations where the chemicals being tested and having similar structures did not show similar toxicity. However, in the evaluation case studies conducted by Kao, the structural similarity of the target and similar chemicals was examined conjointly with the similarity in toxic response observed during cell-based assays, etc., revealing that the toxicity of any given target chemicals could be predicted by inference. This finding effectively resolves the inherent issue in read-across and helps improve the accuracy of inference-based prediction.
These reports compiled by Kao are expected to positively contribute to the OECD IATA Case Studies Project for issuing guidance on new alternative methods to animal testing.
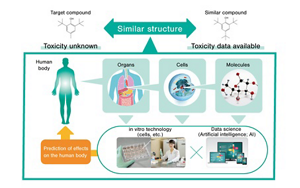
Multi-factor Systemic Toxicity Evaluation Through IATA-based Read-across, Without Involving Animal Testing
About Kao
Kao creates high-value-added products that enrich the lives of consumers around the world. Through its portfolio of over 20 leading brands such as Attack, Bioré, Goldwell, Jergens, John Frieda, Kanebo, Laurier, Merries and Molton Brown, Kao is part of the everyday lives of people in Asia, Oceania, North America and Europe. Combined with its chemical division, which contributes to a wide range of industries, Kao generates about 1,500 billion yen in annual sales. Kao employs about 33,000 people worldwide and has 130 years of history in innovation. Please visit the Kao Group website for updated information.
Media inquiries should be directed to:
Corporate Communications
Kao Corporation
