New Technique to Evaluate the Inactivation Effect of Chemical Formulations on Human Noroviruses
The Safety Science Research Laboratories and the Household Products Research Laboratories of Kao Corporation (President: Michitaka Sawada), in collaboration with Professor Kazuhiko Katayama (Laboratory of Viral Infection I, Omura Satoshi Memorial Institute, Kitasato University) and Professor Toshiro Sato (Mitsunada Sakaguchi Memorial Laboratories, Keio University School of Medicine), have developed a new technique to evaluate the inactivation effect*1 of chemical formulations on human noroviruses. These evaluations have been carried out using noroviruses which are present in human populations instead of the more commonly used laboratory-based viruses from cats and mice.
Using this new technique, the researchers demonstrated that some oxygen-based bleaching mixtures containing sodium percarbonate, surfactant, and bleaching activator, as well as sodium hypochlorite, inhibited the proliferation of human noroviruses. These results support previous reports suggesting the inactivation effect of sodium hypochlorite and demonstrate new evidence of inactivation of human noroviruses by these mixtures. These findings were presented at the 140th Annual Meeting of the Pharmaceutical Society of Japan held from March 25th to 28th, 2020 in Kyoto, Japan.
-
* 1 Inactivation is a process that destroys the infectious ability of the virus.
Background
Noroviruses, which are usually most prevalent in the winter, are a major cause of food poisoning. These viruses are transmitted via contact with contaminated hands or foods, and proliferate in the human gastrointestinal tract, causing symptoms such as vomiting, diarrhea, and abdominal pain. According to a survey conducted by the Ministry of Health, Labour and Welfare of Japan, noroviruses were the most common cause of food poisoning reported in Japan in 2018. Globally, many people develop food poisoning caused by noroviruses and their prevalence is particularly high in developing countries.
Since there is currently no cure or vaccine for norovirus infection, the development of disinfectants or agents that can inactivate noroviruses is essential to prevent food poisoning as well as further spread of the virus.
The development of effective disinfectants for human noroviruses has been difficult as there is no technology available which allows inactivation to be directly verified using in vitro cultures of noroviruses. Therefore, feline caliciviruses (FCVs) and murine noroviruses (MNVs), which cause infection in cats and mice, respectively, and are related to human noroviruses, have been used as alternatives to evaluate disinfectants for their inactivation effects on human noroviruses. However, it has been reported that the characteristics of FCVs and MNVs differ from human noroviruses as they are more easily inactivated under acidic conditions and with alcohol, respectively.
In 2016, it was reported that human noroviruses were successfully cultured using intestinal organoids.*2 Building on this success, another group also succeeded in evaluating the effect of inactivation agents comprised of a single ingredient (e.g., sodium hypochlorite) using human noroviruses.
-
* 2 Intestinal organoids are small three-dimensional tissues that are similar in structure to human intestinal epithelial tissue. They are expected to be utilized in the research of intestinal bacteria and intestinal diseases. They are also known as enteroids.
Research team develops a technique to evaluate the inactivation effect of chemical formulations comprised of two or more ingredients on human noroviruses
To evaluate the effectiveness of inactivating agents on noroviruses, a sample containing an inactivating agent and noroviruses is mixed and allowed to react. This mixture is then added to intestinal organoids to cause viral infection. Following this, a polymerase chain reaction assay is performed to examine whether the copy number of the human norovirus gene has increased. If it has not increased, the agent being tested is considered to have inactivated the human noroviruses.
Most of the commercially available inactivating agents are a mixture comprised of two or more ingredients. Some of these products contain ingredients that adversely affect intestinal organoids (e.g., surfactants). Therefore, it has been difficult to evaluate the inactivation effect of these agents on human noroviruses due to the damage which is incurred by the intestinal organoids. To solve this problem, Kao undertook collaborative research with Kitasato University and Keio University to develop a technique that allowed for the evaluation of the inactivation of noroviruses in a way that does not damage intestinal organoids.
Through a trial and error process, the researchers found ingredients that adversely affect intestinal organoids can be separated from the test samples by adding serum components*3 that enable separation by ultracentrifugation.*4 They found that this approach prevented intestinal organoid damage (Figure 1), and therefore succeeded in developing the first ever technique*5 to evaluate the inactivation effect of complex chemical formulations on human noroviruses.
-
* 3 It is believed that the various kinds of proteins present in serum components contribute to the absorption of molecules that are harmful to intestinal organoids.
-
* 4 Ultracentrifugation is a technique used to separate components of a mixture through the application of centrifugal force at a speed of tens of thousands of rotations per minute.
-
* 5 PubMed, a searchable database of medical and scientific evidence and literature, was searched using the terms "human norovirus," and "organoid or enteroid" and "inactivation or inactivated." There were no search results regarding the inactivation effect of chemical formulations comprised of multiple ingredients (search date: June 1, 2020).
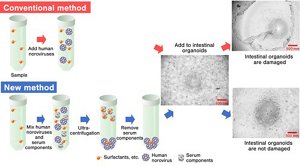
Figure 1. Experiment flow
Oxygen-based bleaching mixture inactivate human noroviruses
Using this newly developed technique, researchers at Kao evaluated the inactivation effect of various kinds of formulations and compounds. They judged that a formulation or compound effectively inactivated human noroviruses if viral proliferation was inhibited, as untreated human noroviruses proliferate in intestinal organoids.
This study confirmed that treatment with sodium hypochlorite, a component of chlorine bleach, at 37°C for greater than one minute inhibited the proliferation of noroviruses. Furthermore, they also demonstrated that oxygen-based bleaching mixture containing sodium percarbonate, surfactant, and bleaching activator were also effective in inhibiting the proliferation of the viruses when the viruses were treated at 37°C for greater than 10 minutes (Figure 2).
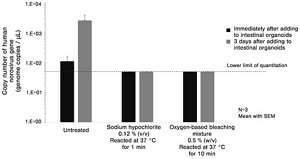
Figure 2. Inactivation effect on human norovirus
Future perspective
Kao continues to contribute extensively to society through the development of technologies against human noroviruses by utilizing this newly developed technique to evaluate products for their inactivating effects on human noroviruses.
About Kao
Kao creates high-value-added products that enrich the lives of consumers around the world. Through its portfolio of over 20 leading brands such as Attack, Bioré, Goldwell, Jergens, John Frieda, Kanebo, Laurier, Merries and Molton Brown, Kao is part of the everyday lives of people in Asia, Oceania, North America and Europe. Combined with its chemical division, which contributes to a wide range of industries, Kao generates about 1,500 billion yen in annual sales. Kao employs about 33,000 people worldwide and has 130 years of history in innovation. Please visit the Kao Group website for updated information.
Media inquiries should be directed to:
Corporate Communications
Kao Corporation
