Prediction of Complex Systemic Toxicity Without Use of Experimental Animals
Predictive Toxicology
For providing safe and secure products to consumers, it is vital to determine in detail the safety of the materials to be used. On the other hand, it is also important to minimize the burden of safety testing. In an effort to improve the lifestyle of consumers through elaborate safety assessments, Kao has been advancing development of evaluation methods for products and raw materials without use of experimental animals.
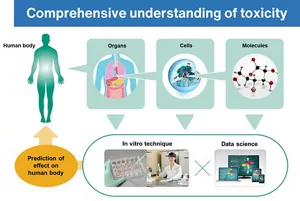
It is considered difficult to predict the various systemic effects of chemical substances on the human body using only in vitro testing, as it is also necessary to examine the effects on multiple organs. We have utilized various approaches, such as a computer-assisted technique for examining toxicity on the basis of the chemical structure of a material and existing toxicological information, as well as in vitro techniques for detailed analysis of the effects on organs using biological samples and cultured cells. In order to solve these difficult problems, understanding of the systemic effects from micro to macro levels is key.
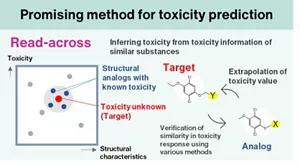
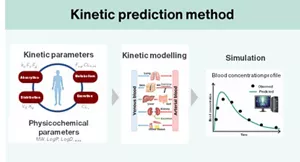
Currently, a predictive technique for assessing the systemic toxicity of these technologies combines the “read-across” toxicity prediction method, which infers the toxicity of a substance from existing toxicological information of chemically and biologically similar substances, with simulation methods that predict the physiological kinetics of substances exposed to the body. These integrated approaches are being utilized for risk assessment based on a comprehensive understanding of toxicity.
The knowledge and research findings obtained from this research have contributing to create a new framework for international next-generation risk assessment through discussions with toxicology experts, various industry associations, and regulatory authorities.
- Home
- Innovation
- Research & Development
- Fundamental Research
- Safety Science
- Prediction of Complex Systemic Toxicity Without Use of Experimental Animals
- Home
- Innovation
- Research & Development
- Fundamental Research
- Safety Science
- Prediction of Complex Systemic Toxicity Without Use of Experimental Animals
